A new analysis published in The Lancet Microbe shows how the SARS-CoV-2 virus spreads from the nose to the air and surfaces in the immediate surroundings. The findings are the second batch of results to come from the COVID-19 Human Challenge Program, led by Imperial College London and partners, and provide granular insights into how people infected with SARS-CoV-2 spread the virus to their immediate surroundings. In February 2021, 36 healthy, young participants with no previous immunity to the virus were infected with SARS-CoV-2 under controlled clinical conditions in a residential facility at the Royal Free Hospital in London, where they could be carefully monitored. They remained at the facility until they were no longer infectious. The facility enabled researchers to track the course of infection in great detail, with the clinical team taking daily swabs from participants’ noses and mouths, as well as environmental samples of the air and swabs of surfaces in their rooms.
Viral emissions
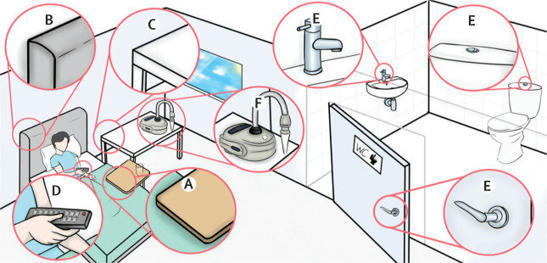
Out of 36 initial participants, a total of 18 became infected. Analysis showed that two individuals emitted substantially more virus into the air than the other infected participants, but displayed no significantly worse symptoms. The researchers suggest this may represent the small proportion of individuals who have potential to be highly infectious, sometimes described as “superspreading.” Analysis found large amounts of viral RNA in air samples, in exhaled breath, as well as swabs of participants hands and on surrounding surfaces, including frequently touched surfaces such as door handles and TV remote controls, showing how an infected person contaminates their surrounding environment and can spread the virus. Viral emissions correlated strongly with the level of virus detected in people’s noses, more than in their throat, highlighting the nose as a significant route for infected people shedding virus into the air and environment. According to the researchers, the latest analysis further highlights the routes by which the virus is transmitted from person to person—directly into the air, depositing onto nearby surfaces, and transferred from contaminated hands to frequently touched surfaces, such as door handles and remote controls.
Reliable indicators
They also show that positive lateral flow tests and visible symptoms were reliable indicators of when people were infectious and emitting virus into the air and environment. The vast majority of virus was emitted after people noticed their first symptoms, with very little virus released into the environment before that (pre-symptomatically). They found no significant link between the severity of participants’ symptoms and the amount of virus they shed into the environment. According to the researchers, the findings highlight the need to reinforce public health messaging around proper face mask use, hand washing and surface cleaning, as well as how human challenge studies continue to add to our knowledge of COVID-19 infection and transmission. Dr. Anika Singanayagam, NIHR Academic Clinical Lecturer in the Department of Infectious Disease at Imperial College London, and joint first author of the study, said, “Our latest findings add to the existing body of knowledge on COVID-19 transmission. By studying infection in a controlled environment, we can collect unique, detailed measurements of virus emitted that allow us to understand how and when people with COVID-19 are contagious to others. These types of measurements are challenging to collect in real-world studies. “Our data indicate that much of the virus people shed comes from the nose, further highlighting the importance of face masks covering the nose as well as the mouth when they’re used. But it also shows how virus can be transferred from hands to contaminate surfaces, like door handles or remote controls, which become a source of infection.”
Dr. Jay, Jie Zhou, research associate in the Department of Infectious Disease at Imperial College London, and joint first author of the study, said, “Understanding when infected people are contagious and how to detect when they are contagious is important—it can help us to use interventions like face masks or social distancing more effectively. The data in our study highlights that being aware of, and acting on, the first minor symptoms that signal an infection, coupled with frequent self-testing with lateral flow tests, can effectively reduce onward spread.” Professor Wendy Barclay, head of the Department of Infectious Disease at Imperial College London, said, “One of the most important things we need to know for controlling the spread of respiratory viruses, such as SARS-CoV-2, is when are people who are actively infected with the virus most likely to infect others? That information can help to tell us how the virus will spread, and how best to use interventions to stop the outbreak. “Human challenge studies enable us to gain granular insights into the infection which we might not otherwise be able to. They play an important role in our understanding of infectious diseases, and should be considered a part of future pandemic preparedness.”
Previous findings
The latest findings add to several key clinical insights already gained from the COVID-19 Human Challenge Program, published in February 2022. These include that symptoms start to develop on average two days after contact with the virus, that infection first appears in the throat, infectious virus peaks about five days into infection and, at that stage, is significantly more abundant in the nose than the throat. The first analysis also found that lateral flow tests (LFTs) are a reassuringly reliable indicator of whether infectious virus is present in the nose and throat (i.e., whether they are a likely to be infectious to other people).
Original research published (June 9, 2023) at The Lancet Microbe:
https://doi.org/10.1016/S2666-5247(23)00101-5