Terrible to terrific: A new antifungal molecule tweaks a powerful drug to harness its power against infection while doing away with its toxicity.
A new antifungal molecule, devised by tweaking the structure of prominent antifungal drug Amphotericin B, has the potential to harness the drug’s power against fungal infections while doing away with its toxicity, researchers at the University of Illinois Urbana-Champaign and collaborators at the University of Wisconsin-Madison report in the journal Nature.
Amphotericin B, a naturally occurring small molecule produced by bacteria, is a drug used as a last resort to treat fungal infections. While AmB excels at killing fungi, it is reserved as a last line of defense because it also is toxic to the human patient – particularly the kidneys.
“Fungal infections are a public health crisis that is only getting worse. And they have the potential, unfortunately, of breaking out and having an exponential impact, kind of like COVID-19 did. So let’s take one of the powerful tools that nature developed to combat fungi and turn it into a powerful ally,” said research leader Dr. Martin D. Burke, an Illinois professor of chemistry, a professor in the Carle Illinois College of Medicine and also a medical doctor.
“This work is a demonstration that, by going deep into the fundamental science, you can take a billion-year head start from nature and turn it into something that hopefully is going to have a big impact on human health,” Burke said.
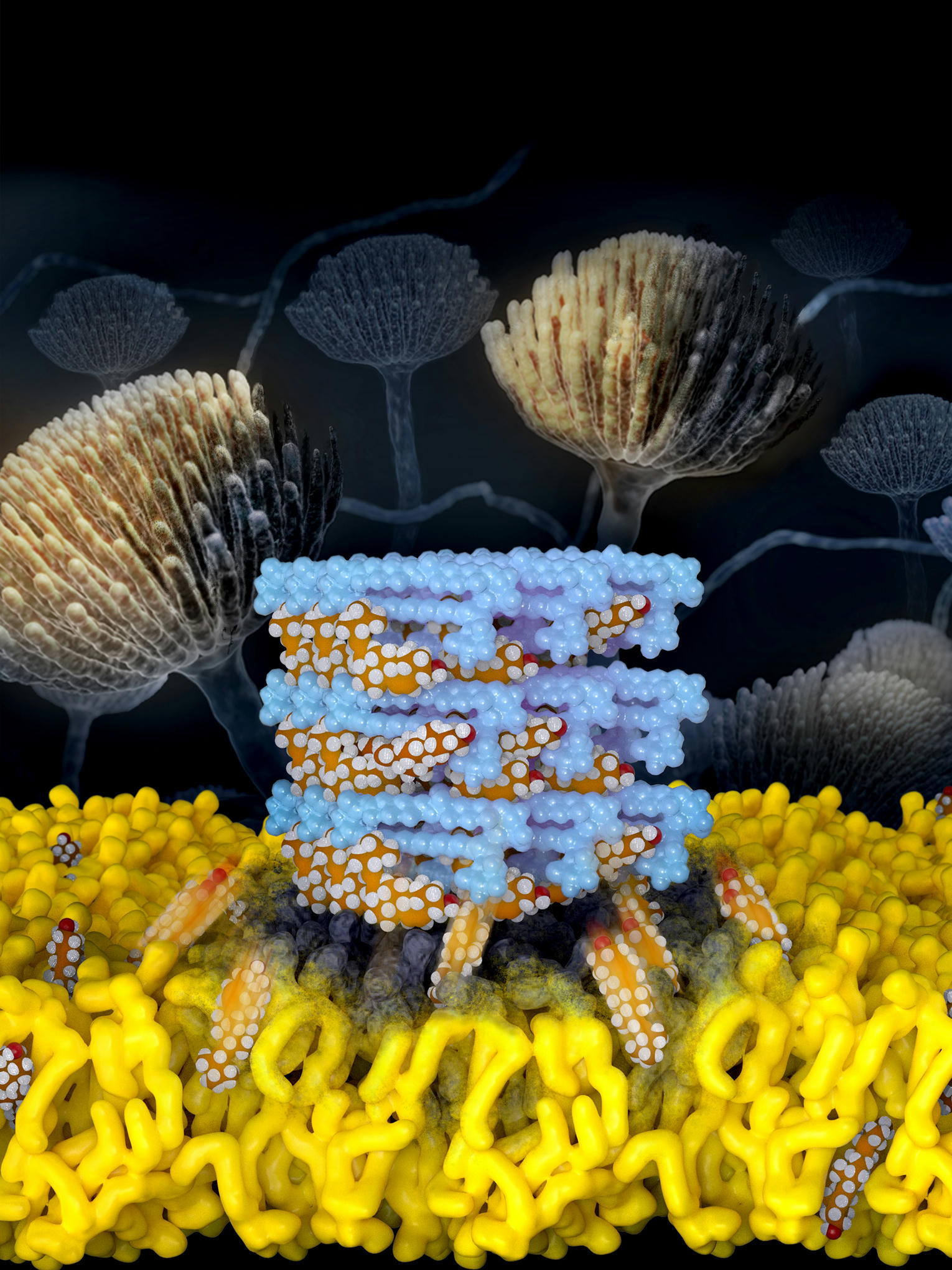
Burke’s group has spent years exploring AmB in hopes of making a derivative that can kill fungi without harm to humans. In previous studies, they developed and leveraged a building block-based approach to molecular synthesis and teamed up with a group specializing in molecular imaging tools called solid-state nuclear magnetic resonance, led by professor Chad Rienstra at the University of Wisconsin-Madison. Together, the teams uncovered the mechanism of the drug: AmB kills fungi by acting like a sponge to extract ergosterol from fungal cells.
In the recent work, Burke’s group worked again with Rienstra’s group to find that AmB similarly kills human kidney cells by extracting cholesterol, the most common sterol in people. The researchers also resolved the atomic-level structure of AmB sponges when bound to both ergosterol and to cholesterol.
“The atomic resolution models were really the key to zoom in and identify these very subtle differences in binding interactions between AmB and each of these sterols,” said Illinois graduate student Corinne Soutar, a co-first author of the paper. “Using this structural information along with functional and computational studies, we achieved a significant breakthrough in understanding how AmB functions as a potent fungicidal drug,” Rienstra said. “This provided the insights to modify AmB and tune its binding properties, reducing its interaction with cholesterol and thereby reducing the toxicity.”
Read the full article at: news.illinois.edu